What is the Lewis Structure of H2CO?
What is the Lewis Structure of H2CO? The Lewis structure has carbon as the central atom with single bonds to both chlorine atoms and a double bond to the oxygen atom. There are two lone pairs of electrons on the oxygen atom and three lone pairs on each chlorine atom.
What is this molecule and what is it used for?
H2CO is a molecule that you probably already know from biology class as formaldehyde. it has a number of applications, outside of the most common use, which is embalming tissue for preservation. It is also used as a resin in construction, a preservative/disinfectant, as a textile treatment and in manufacturing chemicals and pharmaceuticals.
Method 1: Step method to draw the Lewis structure of H2CO.
In this method, we find the bonds and lone pairs for the whole molecule, then plug it in to the atoms that we have to get the answer. Here is a little flow chart of how we are going to do this:
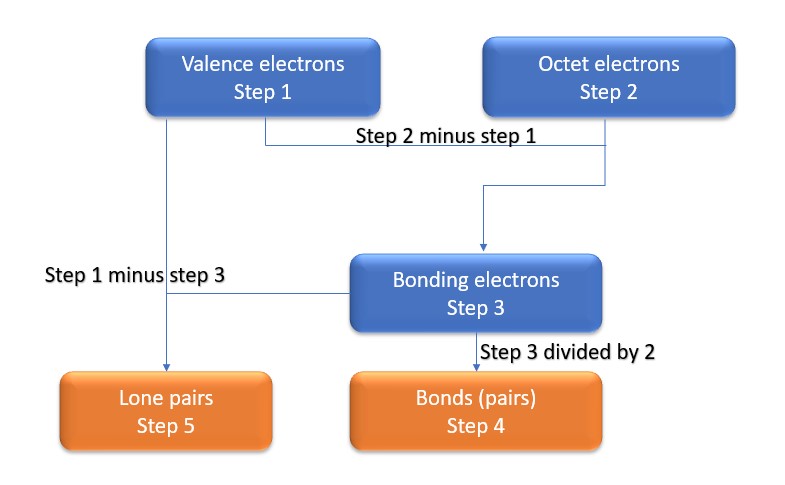
We will go through the steps below, but one thing to note here is that all the valence electrons (step 1) are either lone pairs OR bonding electrons. In other words…. Lone Pairs (Step 5) + Bonding electrons (Step 3) = Valence electrons (Step 1) . Let’s go through this example so we can see this a little more clearly.
Step 1: Find valence electrons for all atoms. This is determined by looking at which column on the periodic table the atom is in, ignoring the transition metals in the middle. Add the valence electrons for each atom together.
C : 1×4 = 4
H : 2×1 = 2
O : 1×6 = 6
Total = 12 valence electrons
Step 2: Find octet electrons for each atom and add them together. Most atoms like 8 electrons to form an octet, however hydrogen is one of the exceptions, as it only wants two electrons to form an octet.
C: 1×8 = 8
H: 2×2 = 4
O: 1×8 = 8
Total = 20 “octet” electrons
Step 3: Find the number of bonding electrons. Subtract the valence electrons (step 1) from the octet electrons (step 2). This gives the number of bonding electrons.
20-12=8 bonding electrons.
Step 4: Find number of bonds by diving the number of bonding electrons (step 3) by 2 because each bond is made of 2 e-
8 bonding electrons/2 = 4 bond pairs
Step 5: Find the number of nonbonding (lone pairs) electrons. Subtract bonding electrons (step 3) number from valence electrons (step 1).
12 valence -8 bonding = 4 electrons = 2 lone pair
Now, use the information from step 4 and 5 to draw the Lewis structures. Remembering too (this is important):
Neutral carbon has four bonds and no lone pairs
Hydrogen has one bond and no lone pairs
Neutral oxygen has two bonds and two lone pairs.
[Note: For more information on the natural state of common atoms, see the linked post here.]
The carbon atom will be our central atom because it is the most electropositive. We then place the hydrogen and oxygen atoms around it, creating enough bonds to each to ensure an octet:
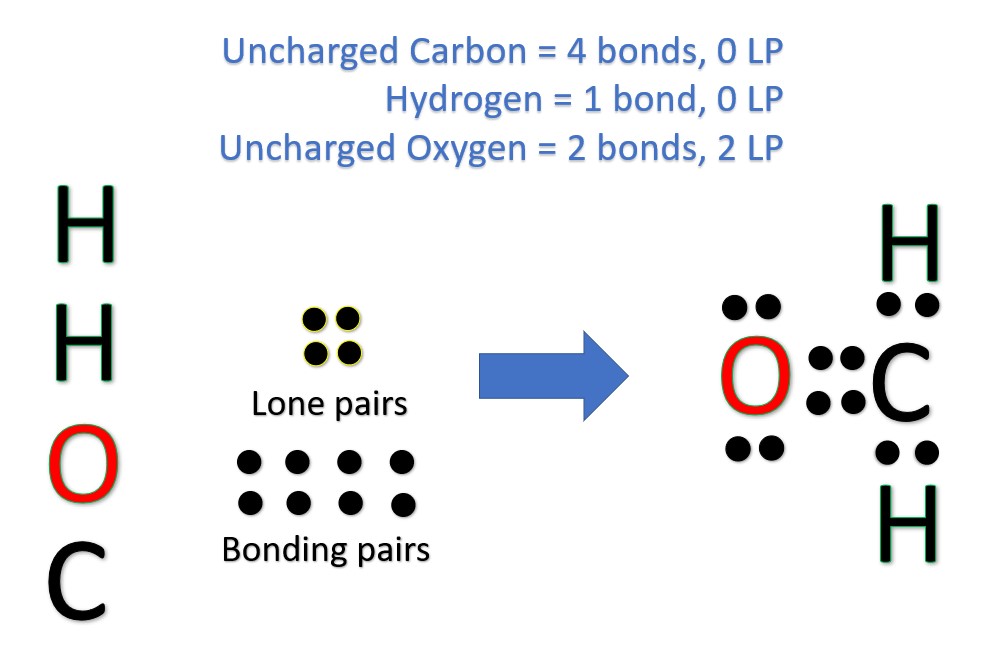
Another (easier) method to determine the Lewis structure of H2CO:
Alternatively a dot method can be used to draw the Lewis structure.
Calculate the total valence electrons in the molecule.
C : 1×4 = 4
H : 2×1 = 2
O : 1×6 = 6
Total = 12 valence electrons
Now, treat the atoms and electrons like puzzle pieces. Put carbons in the center and arrange hydrogen and oxygen atoms on the sides. Remember the natural state of each atom, as discussed above. [Oxygen: 2 bonds, 2 LP; Hydrogen: 1 bonds, 0 LP; Carbon: 4 bonds, 0 LP] Also, make sure sure each atom has an octet, which in the case of hydrogen is only two electrons.

Frequently asked questions:
Q: So what is the difference between the two methods?
A: In the first method, we are figuring out all of the lone pairs and bonds first, then placing those electrons and bonds on the atoms to form a molecule. In the puzzle method, we already have lone pairs and bonding electrons assigned to each atom, so all we need to do is push puzzle pieces together to get a molecule. In each method, we need to remember the “happy state” of each atom, ei hydrogen likes 1 bond and no lone pairs, uncharged carbon likes four bonds and no lone pairs ect.
Q: What is going on with the double bond between oxygen and carbon?
A: A double bond between oxygen and carbon is called a carbonyl. It is electrophilic (meaning it has a small, partial positive charge) and reactive at the carbon atom. With respect to Lewis dot structures, the four electrons between the carbon and the oxygen count as four electrons for both O and C in satisfying the octet rule.
And now some video:
This is a quick video we put together that visually demonstrates the two methods for Lewis structure and Lewis dot problems.
And finally, the Lewis structure study guide:
Here it is, this is our one-page guide to Lewis Dot and Lewis Structures:

